Course info
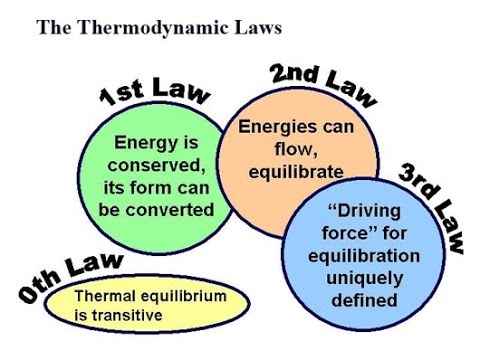
First law of thermodynamics: Work and heat. Heat capacity. Work of expansion and compression. Isothermal and adiabatic process. Second law of thermodynamics: spontaneous process. Definition of entropy and thermodynamic. Entropy change, entropy as a function of temperature and volume, entropy as a function of temperature and pressure. Third law of thermodynamics: Absolute entropy. Spontaneity Equilibrium. Thermodynamics of open system. Electrochemistry: References: 1. Thomas Engel and Philip Reid, (2006). Thermodynamics, Statistical Thermodynamics and Kinetics. Pearson International Edition. 2. David W Ball (2003). Physical Chemistry. Thomson Learning, Inc. 3. I.N.Levine (2002). Physical Chemistry, (5th Edition). McGraw Hill.
- Lecturer: PUAN ANIS TASNIM MD. YUSOF
Social networks